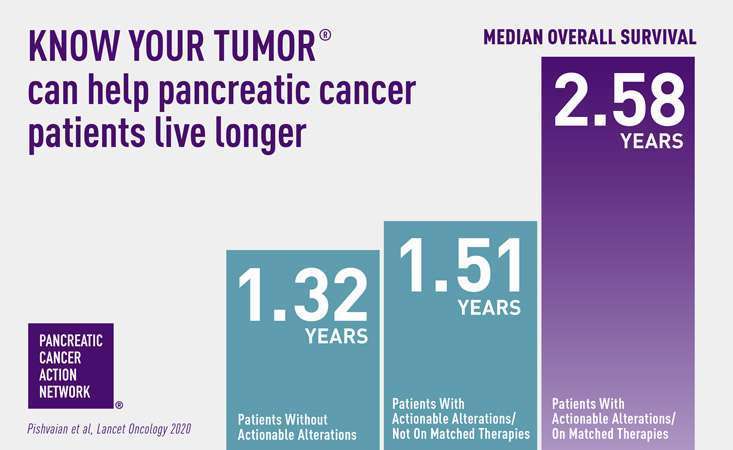
Patients treated with matched therapies selected through biomarker or genetic testing can live longer1. The Pancreatic Cancer Action Network (PanCAN) supports guideline recommendations for all patients to undergo genetic testing for inherited mutations at diagnosis and for patients to undergo biomarker testing of their tumor tissue unless clinically contraindicated. PanCAN offers precision medicine information and resources, including our Know Your Tumor® precision medicine service, for you and your patients.
1PanCAN's Public Statements for Healthcare Professionals, including references.
Valuable Information to Support Your Treatment Decisions
PanCAN urges healthcare professionals to provide pancreatic cancer patients with genetic testing for inherited mutations at diagnosis and biomarker testing of their tumor tissue unless clinically contraindicated. If needed, our Know Your Tumor service is available to you and your patients.
PanCAN’s Know Your Tumor precision medicine service is provided in partnership with Tempus, a CLIA-certified laboratory. The service provides you with a report of your patient’s tumor and germline biology and treatment options, providing valuable insight to support your treatment decisions.
More than 400 physicians from 49 states have received Know Your Tumor reports for their patients. About 26% of patients’ tumors have been found to have at least one actionable alteration.
Contact PanCAN Patient Services for more information.
“Know Your Tumor is a very important effort to improve the care provided to pancreatic cancer patients. We gain an important understanding of tumor biology, which leads to more precise selection of treatments. This is personalized medicine at its best.”
— Dr. Andrew E. HendifarMedical oncology lead for the gastrointestinal disease research group at Cedars-Sinai Medical Center
More Treatment Options
The Know Your Tumor report includes treatment options, providing valuable insight to support your treatment decisions.
Treatment options, personalized to your patient, may include:
Targeted therapy, which avoids the toxicity of multi-agent chemotherapy
Appropriate clinical trials, including molecularly targeted solid tumor trials
Off-label treatments that may be particularly effective for your patient’s specific tumor mutations
Standard of care treatments
Our Trusted Partners
PanCAN partners with Tempus, a CLIA-certified laboratory that uses the xT solid tumor gene panel or the xF liquid biopsy gene panel for patients with quantity non-sufficient (QNS) samples for testing. The report includes information on both somatic and germline mutations, tumor burden (TMB) and microsatellite instability (MSI).
PanCAN partners with Perthera, Inc., to publish research supported by data from the Know Your Tumor program. These efforts will help improve patient outcomes by providing important research findings to the healthcare professional community.
The Know Your Tumor Process




Eligibility
The following criteria will help you decide which patients are best suited for Know Your Tumor:
Patient has diagnosis of pancreatic malignancy
Tumor tissue is obtainable (see TEMPUS Specimen Guidelines)
Patient is receiving treatment for a pancreatic cancer diagnosis in the United States and is not imprisoned
Patient or close caregiver reads and speaks English or Spanish
Patient is unable to access testing due to geography, insurance coverage, cost or other factors.
Know Your Tumor Resources
We're Here to Help
PanCAN Patient Services has dedicated staff to provide free, personal one-to-one support for healthcare professionals.