
Adaptive Clinical Trial Design Is More Cost Effective, Patient-Centric and Allows for Faster Learning Than Standard Trials
Los Angeles, Calif. – (Oct. 13, 2020) The Pancreatic Cancer Action Network (PanCAN) announced today the launch of Precision PromiseSM, the first of its kind adaptive clinical trial platform for pancreatic cancer patients. PanCAN’s Precision Promise was envisioned with a nationwide team of leading clinicians, researchers, and diagnostic and drug developers to test novel treatment options for pancreatic cancer patients quicker and cheaper and get them to patients faster, transforming the way clinical research is done.
Every pancreatic cancer treatment available today was approved through a clinical trial; however, standard trials are slow, costly, and have only had a 10 percent success rate over the last 20 years. PanCAN’s novel clinical trial platform is designed to enable the development of new treatments more efficiently than standard pancreatic cancer trials by testing multiple experimental therapies at the same time. Through Precision Promise, metastatic pancreatic cancer patients may have the opportunity to receive both first- and second-line treatment options in one clinical trial. As a late-stage platform, the potential for new drug approvals by the FDA is built into the model, which can accelerate the drug development process by up to two years. The statistical design of Precision Promise was led by world-renowned statistician Dr. Donald Berry (Berry Consultants), who designed the I-SPY breast cancer trials and has over 400 peer-reviewed publications.
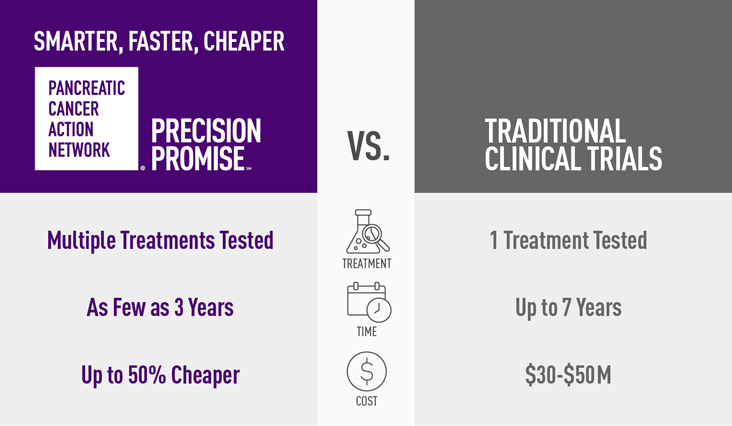
Pancreatic cancer is currently the third leading cause of cancer-related death in the U.S., with an overall five-year survival rate of just 10 percent. Roughly 63 percent of patients die within the first year of a pancreatic cancer diagnosis, underscoring the urgent need for new and more effective treatment options.
“Pancreatic cancer patients don’t have time to wait for new treatments to be approved through the standard clinical trial model,” said Julie Fleshman, JD, MBA, PanCAN’s president and CEO. “As the first pancreatic cancer nonprofit organization to develop, sponsor, and lead an adaptive clinical trial platform, we are disrupting the current clinical trial system in order to accelerate progress for patients fighting this disease.”
Eligible pancreatic cancer patients will be able to enroll in PanCAN’s Precision Promise at one of 15 Clinical Trial Consortium sites nationwide. Sites were selected through a competitive, peer-review process and include:
- Cedars-Sinai Medical Center (Los Angeles, Calif.)
- Dana-Farber/Harvard Cancer Center (Boston, Mass.)
- Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance/University of Washington (Seattle, Wash.)
- Johns Hopkins Medicine (Baltimore, Md.)
- Memorial Sloan Kettering Cancer Center (New York City, N.Y.)
- Moores Cancer Center at UC San Diego Health (San Diego, Calif.)
- Perelman School of Medicine University of Pennsylvania (Philadelphia, Pa.)
- Perlmutter Cancer Center/NYU Langone Health (New York City, N.Y.)
- The University of Chicago (Chicago, Ill.)
- The University of Texas MD Anderson Cancer Center (Houston, Texas)
- UC San Francisco Helen Diller Family Comprehensive Cancer Center (San Francisco, Calif.)
- University of Florida Health – Cancer Center (Gainesville, Fla.)
- Virginia Mason Medical Center (Seattle, Wash.)
- Washington University School of Medicine (St. Louis, Mo.)
- Weill Cornell Medicine (New York City, N.Y.)
A full list of institutions that are open and actively enrolling patients can be found at pancan.org/precisionpromise/locations.
In partnership with Tempus, a leader in artificial intelligence and precision medicine, every patient enrolled in PanCAN’s Precision Promise trial will undergo broad-panel genomic testing. Precision promise will also support follow-up biopsies to learn how their tumor is responding to treatment. Through the adaptive nature of PanCAN’s Precision Promise, data will be constantly monitored, and treatment arms can be discontinued if results do not look promising.
“By designing a more patient-centric trial platform, we are able to test promising new therapies more quickly and learn from fewer patients if a treatment is working,” said Diane Simeone, MD, chair of the PanCAN Precision Promise Steering Committee and Precision Promise Principal Investigator at Perlmutter Cancer Center at NYU Langone Health. “The patients that enroll will also receive best-in-class supportive care alongside treatment with biomarker testing to better understand why treatments work in some patients but not in others. This approach should serve as a model for the next generation of trials – not only for pancreatic cancer, but for all diseases.”
PanCAN is the trusted and unbiased leader that is bringing together key stakeholders – investigators, clinicians, industry, pancreatic cancer thought-leaders and regulatory authorities – to make Precision Promise possible.
“Through groundbreaking initiatives like Precision Promise, PanCAN is committing to taking bold action in the fight against pancreatic cancer,” said Hilarie Koplow-McAdams, Venture Partner at New Enterprise Associates and chair of PanCAN’s Board of Directors, who lost her father to pancreatic cancer in 2012. “Anyone who has been personally touched by this disease understands the urgency needed to develop new treatment options. With PanCAN taking the initiative to drive innovation in this space, we can make meaningful progress.”
PanCAN is grateful to its generous donors as well as its Scientific & Medical Affairs Industry Members who help make research initiatives like Precision Promise possible. Industry members include: AbbVie, AstraZeneca, IPSEN, Pfizer, Rafael Pharmaceuticals, Tempus, TriSalus Life Sciences, and Tyme Technologies, Inc.
To learn more about Precision Promise and PanCAN’s commitment to research, visit pancan.org. Pancreatic cancer patients and caregivers can receive personalized support and resources, and more information about Precision Promise, through PanCAN’s Patient Services or by calling 877-2-PANCAN.
For the latest organization updates, follow the PanCAN on Facebook, Twitter, and Instagram.















