
— Julie Fleshman, JD, MBA, PanCAN President and CEO
Smarter, faster, cheaper.
Constantly learning and adapting. A patient-first approach. Findings shared with researchers everywhere. Benefits everyone facing pancreatic cancer.
This is PanCAN’s Precision PromiseSM, the groundbreaking new approach to pancreatic cancer clinical trials, created to speed progress for patients by getting new and better treatments approved more quickly. So that in the future, patients are no longer advised to, “Go home and get your affairs in order.”
And today, after collaboration with more than 100 expert clinicians, researchers and diagnostic and drug developers, and with generous funding from visionary donors, PanCAN’s Precision Promise is open to eligible patients to enroll at 12 world-class cancer institutions nationwide, with more sites opening soon.
Why Precision Promise Is a Game-Changer
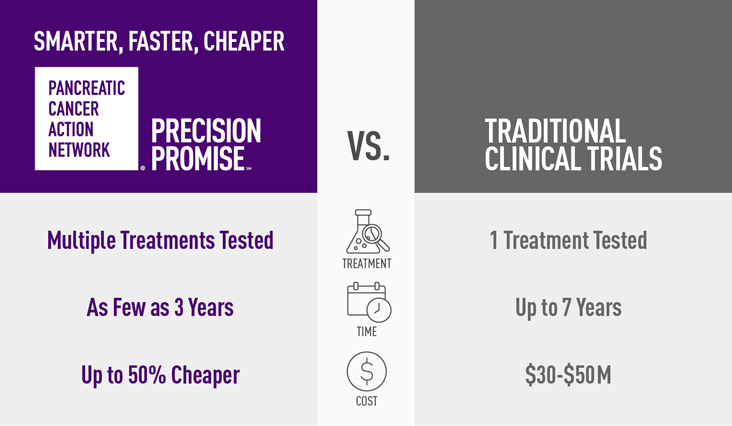
Clinical trials are the only way to get new treatments approved, but the standard system does not bring new treatments to patients quickly enough.
Standard clinical trials:
- Test one treatment at a time
- Can take many years to learn whether the treatment is effective
- Are very expensive
- Give patients one treatment option while enrolled in the trial
- Require as many as 1,000 patients to enroll, and results aren’t known until the end of the trial
In the last 20 years, the success rate for new pancreatic cancer treatments has been low (just 10%), making for a risky drug development space for pharmaceutical companies.
Precision Promise is different than standard clinical trials in that it has an adaptive design, meaning if a drug is not working, it can be pulled from the trial and another treatment can take its place. And if a drug is working, it can move more quickly to the FDA for potential approval.
Also, multiple treatments can be tested at the same time, and only 175 patients are needed in order to know if a treatment is working. This makes for a much cheaper clinical trial for pharmaceutical companies.
PanCAN’s Precision Promise is:
- Smarter – Clinicians learn from every patient every step of the way, making modifications throughout the trial
- Faster – Researchers will learn in two to four years, not five to seven as with standard clinical trials
- Cheaper – Up to 50% less expensive than a traditional clinical trial
PanCAN President and CEO Julie Fleshman, JD, MBA, understands the need, saying, “As the first pancreatic cancer nonprofit to develop, sponsor and lead an adaptive clinical trial platform, we are disrupting the current clinical trial system to accelerate progress for patients. They simply don’t have time to wait.”
Who Is Eligible to Enroll
Patients with metastatic pancreatic cancer who have not yet had treatment, or who have received only first-line treatment, may be eligible to enroll in PanCAN’s Precision Promise.
A Patient-First Approach
At PanCAN, we speak with more patients than any other organization in the world, and patients are our top priority.

Members of PanCAN’s Precision Promise steering committee with Julie Fleshman (far left), PanCAN President and CEO. At far right is Diane Simeone, MD, Precision Promise Steering Committee Chair and Principal Investigator at Perlmutter Cancer Center/NYU Langone Health.
Precision Promise was designed for patients and also emphasizes the study of supportive care: tending to the whole patient and addressing quality of life, pain management, symptom and side effect management, activity levels and more.
In addition to studying supportive care measures, PanCAN’s Precision Promise will include comprehensive testing on every enrolled patient to discover future biomarkers for the disease. This information can also help answer research questions that explain why patients respond differently to the same treatment.
And throughout the trial, patients will have follow-up biopsies to learn how their tumor is responding to treatment in real time.
For more information about Precision Promise and other clinical trials, including free, in-depth and personalized resources for pancreatic cancer, contact PanCAN’s Patient Services.
A Benefit to the Entire Pancreatic Cancer Community
PanCAN will also collect secure patient data from all Precision Promise sites and share scientific findings with pancreatic cancer researchers and clinicians nationwide in order to learn and build on it. This will benefit pancreatic cancer patients everywhere.
At PanCAN, our vision is a world where all patients with pancreatic cancer thrive. Where they have new, better treatment options and a better chance of longer survival.
— Diane Simeone, MD, Precision Promise Steering Committee Chair and Principal Investigator at Perlmutter Cancer Center/NYU Langone Health
Next week: The Bold Path to Precision Promise. Read more about how – and why – PanCAN set out to transform traditional clinical trials for pancreatic cancer patients.





