
The Pancreatic Cancer Action Network’s (PanCAN) Know Your Tumor® precision medicine service can help pancreatic cancer patients live longer.
Know Your Tumor was created to understand the unique differences in each patient’s tumor – and to determine whether these differences can impact which treatment options may work best for each patient. Using results from testing a patient’s tumor or genetic makeup to guide treatment decisions is known as precision medicine.
Published today in the prestigious journal Lancet Oncology, data from Know Your Tumor shows for the first time that pancreatic cancer patients who are able to go on therapies that match their tumor biology live an average of one year longer compared to patients who don’t.
When patients enroll in Know Your Tumor, the patient and their healthcare teams receive a detailed report of all the findings within their tumor. The report also lists treatment options for that patient to consider, which may include drugs approved to treat pancreatic cancer, drugs approved to treat other cancer types or experimental therapies that are available through clinical trials.
“Looking at reports from 1,082 patients with pancreatic cancer, we found that one of every four tumors had a change that indicates certain treatment options may work particularly well for that patient,” said Lynn Matrisian, PhD, MBA, PanCAN’s chief science officer and co-author of the study, published in partnership with Perthera, Inc., and other collaborators.
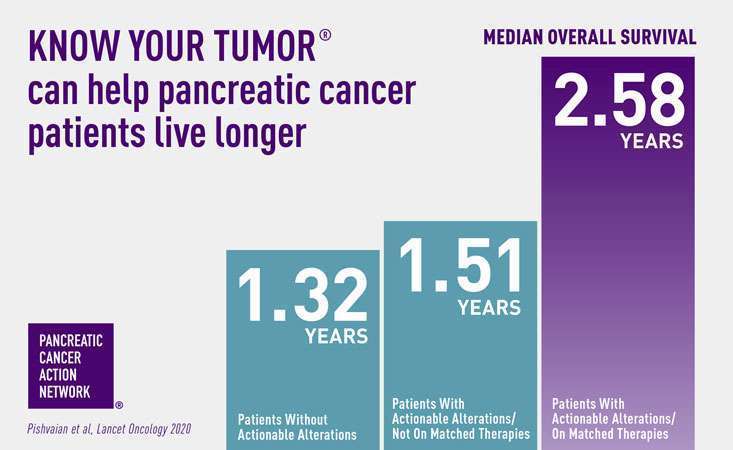
When patients whose tumors had one of these changes went on a treatment option that was listed in their Know Your Tumor report as matching their tumor biology, they lived on average one year longer than patients with similar changes who did not go on a matched therapy or patients whose report did not show any changes that align with a particular treatment option.
“The only way pancreatic cancer patients can know if any of these changes occur in their tumor or their genetic makeup is through testing,” Matrisian said.
There are two types of tests available through Know Your Tumor:
- Tumor molecular profiling, which evaluates a small piece of tumor tissue, often taken during a biopsy, for mutations or other alterations that occurred in the patient’s pancreatic cancer cells.
- Germline (genetic) testing, which analyzes a patient’s saliva or blood for genetic changes they were born with.
PanCAN strongly recommends that all pancreatic cancer patients have both types of tests to help them and their healthcare team make informed treatment decisions. This recommendation is reinforced by guidelines established by the National Comprehensive Cancer Network (NCCN).
“The results from our paper are a strong reminder to healthcare professionals to offer tumor profiling and genetic testing to all their pancreatic cancer patients,” Matrisian said. “And it will further provide incentive to the scientific community to pursue new targeted treatments for even more pancreatic cancer patients.”
The most common changes seen in Know Your Tumor reports were defects in the cancer cells’ ability to repair DNA damage, such as BRCA mutations. Chemotherapies that contain platinum, like oxaliplatin or cisplatin, are particularly effective treatments in patients with these changes.
Pancreatic cancer patients who were born with BRCA mutations may also be eligible to receive Lynparza®, a targeted drug approved in December 2019. Some Know Your Tumor reports provided patients information about clinical trials testing Lynparza and similar drugs.
Other tumor changes predict patients’ response to an immunotherapy drug called Keytruda®, targeted therapies like Vitrakvi® or other standard or experimental treatment options.
The hope for the future is for all pancreatic cancer patients to get tested and know about changes in their tumors or genetic makeup that may inform treatment decisions – and for them to be able to access matched therapies that may help them live longer. Additionally, there is a need for more treatment options to be developed that can benefit additional subsets of pancreatic cancer patients.
“We’re grateful to our research partners and our incredibly generous donors and supporters who made this work possible,” Matrisian said. “Most of all, we’re grateful to the patients and their families who agreed to participate in this service to allow us to gather and publish these pivotal results.”















